 |
 |
 |
ha2xtndA RNA helicase is an enzyme that unwinds dsRNA. The unwinding activity is associated with the hydrolysis of a nucleoside triphospate (NTP), preferentially ATP. However the precise mechanism and the substrates of these enzymes have not been defined, but it is know that the substrate is not only double stranded RNA, it might also be an RNA protein complex (Anderson and Parker 1996) (Linder 2004) The cluster is conformed only by hypothetical proteins or proteins which possess the HA2 domain, which is under the accession number PF04408 in PFAM. Approximately 90 amino acid residues in length constitute this presumed domain. It is found in two different groups of RNA helicases: ATP-dependent helicase HrpA (IPR10222) and ATP-dependent helicase HrpB (IPR10225) going to C-termini of them. Its function is unknown, however it seems likely to be involved in nucleic acid binding. However the matched region in the HA2-containing proteins, overlaps with this domain, and the retrieved sequences redefining the domain (merging the already known domain and the newly matched region) are the same proteins that are now annotated with the HA2 domain. Examples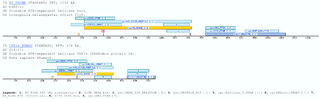
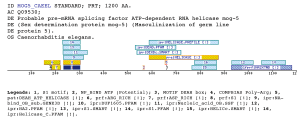

Taxonomic DistributionInsects: Anopheles gambiae, Papilio xuthus, Bombyx mori, Samia cynthia.PSI-BLAST against ensembl (Apis mellifera)
PSI-BLAST against ensembl (no taxonomic restriction)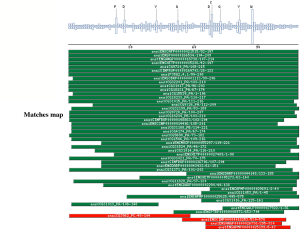
Comparison against UNIPROT
Retrived sequences in UNIPROT (txt format) Downloadable filesProfileMultiple Alignment PSI-BLAST against ensembl (Apis mellifera) PSI-BLAST against ensembl (no taxonomic restriction) ReferencesAnderson, J. S. and R. Parker (1996). "RNA turnover: the helicase story unwinds." Curr Biol 6(7): 780-2. Linder, P. (2004). "Molecular biology. The life of RNA with proteins." Science 304(5671): 694-5. |