 |
 |
 |
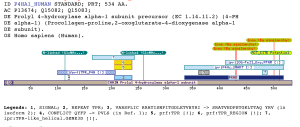
tpr_p4hProlyl 4-hydroxylase catalyzes the hydroxylation of proline in -Xaa-Pro-Gly- triplets in collagens and other proteins with sequences similar to collagen. The enzyme is very important in the synthesis of all collagens, because the 4-hydroxyproline residues formed in the reaction are essential for the folding of the newly synthesized collagen polypeptide chains into triple helical molecules. The vertebrate enzyme is tetramer in which the subunits contribute to most parts of the two catalytic sites (Freedman, Hirst et al. 1994; Helaakoski, Annunen et al. 1995). The subunit is identical to the enzyme protein disulfide-isomerase and has PDI activity even when present in the prolyl 4-hydroxylase tetramer (Kivirikko, Myllyla et al. 1989) (Koivu, Myllyla et al. 1987; Noiva and Lennarz 1992). The PDI polypeptide also has several other functions (Pihlajaniemi, Helaakoski et al. 1987; Parkkonen, Kivirikko et al. 1988). Prolyl 4-hydroxylase had long been assumed to be of one type only, with no isoenzymes (Freedman, Hirst et al. 1994; Helaakoski, Annunen et al. 1995), but an isoform of the subunit, termed the (II) subunit, has been cloned from mouse tissues (Prockop and Kivirikko 1995). Correspondingly, the previously known subunit is now called the (I) subunit. The (II) subunit was found to form an ((II))22 tetramer with the PDI/ subunit when the two polypeptides were coexpressed in insect cells. The properties of the new type II enzyme were found to be very similar to those of the type I tetramer, with the distinct difference that it was inhibited by poly(L-proline) only at very high concentrations (Prockop and Kivirikko 1995). The proposed domain is found exclusively in Prolyl 4-hydrolase alpha related protein also called Procollagen-proline-2-oxoglutarate-4-dioxygenase alpha1 subunit.Furthermore, os always located at the N-ter of SMART :P4Hc and PFAM:2OG-FeII_Oxy (PF03171). The matched region Overlaps with the SSF :TPR-like. No annotations have been added to the region matched by this profile. The tetratrico peptide repeat (TPR) is a motif present in a wide range of proteins (Goebl and Yanagida 1991; Lamb, Tugendreich et al. 1995; Das, Cohen et al. 1998). It mediates protein–protein interactions and is involved in the assembly of multiprotein complexes (D'Andrea and Regan 2003). The TPR motif consists of 3–16 tandem-repeats of 34 amino acids residues, although individual TPR motifs can be dispersed in the protein sequence. TPR motifs have been identified in various different organisms, ranging from bacteria to humans. Proteins containing TPRs are involved in a variety of biological processes, such as cell cycle regulation, transcriptional control, mitochondrial and peroxisomal protein transport, neurogenesis and protein folding. The 2OG-Fe(II) oxygenase superfamily contains members of the 2-oxoglutarate (2OG) and Fe(II)-dependent oxygenase superfamily (Annunen, Helaakoski et al. 1997). This family includes the C-terminal of prolyl 4-hydroxylase alpha subunit. The full enzyme consists of a alpha2 beta2 complex with the alpha subunit contributing most of the parts of the active site (Roach, Clifton et al. 1995). The family also includes lysyl hydrolases, isopenicillin synthases and AlkB. Examples


Taxonomic DistributionVertebrates: Homo sapiens, Mus musculus, Rattus norvegicus, Gallus gallus. Insects: Drosophila melanogaster, Anopheles gambiae. Invertebrates: Caenorhabditis elegans, Caenorhabditis briggsae, Onchocerca volvulus. Plants: Oryza sativa. Bacteria: Corynebacterium equii, Legionella pneumophila Fungi: Saccharomyces cerevisiae. PSI-BLAST against ensembl (Apis mellifera)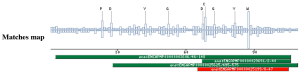
PSI-BLAST against ensembl (no taxonomic restriction)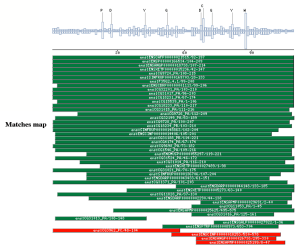
Comparison against UNIPROT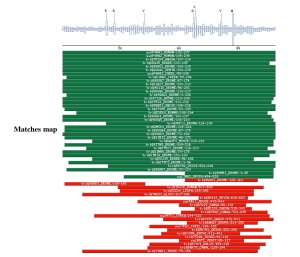
Retrived sequences in UNIPROT (txt format) Downloadable filesProfileMultiple Alignment PSI-BLAST against ensembl (Apis mellifera) PSI-BLAST against ensembl (no taxonomic restriction) REFERENCESAnnunen, P., T. Helaakoski, et al. (1997). "Cloning of the human prolyl 4-hydroxylase alpha subunit isoform alpha(II) and characterization of the type II enzyme tetramer. The alpha(I) and alpha(II) subunits do not form a mixed alpha(I)alpha(II)beta2 tetramer." J Biol Chem 272(28): 17342-8. D'Andrea, L. D. and L. Regan (2003). "TPR proteins: the versatile helix." Trends Biochem Sci 28(12): 655-62. Das, A. K., P. W. Cohen, et al. (1998). "The structure of the tetratricopeptide repeats of protein phosphatase 5: implications for TPR-mediated protein-protein interactions." Embo J 17(5): 1192-9. Freedman, R. B., T. R. Hirst, et al. (1994). "Protein disulphide isomerase: building bridges in protein folding." Trends Biochem Sci 19(8): 331-6. Goebl, M. and M. Yanagida (1991). "The TPR snap helix: a novel protein repeat motif from mitosis to transcription." Trends Biochem Sci 16(5): 173-7. Helaakoski, T., P. Annunen, et al. (1995). "Cloning, baculovirus expression, and characterization of a second mouse prolyl 4-hydroxylase alpha-subunit isoform: formation of an alpha 2 beta 2 tetramer with the protein disulfide-isomerase/beta subunit." Proc Natl Acad Sci U S A 92(10): 4427-31. Kivirikko, K. I., R. Myllyla, et al. (1989). "Protein hydroxylation: prolyl 4-hydroxylase, an enzyme with four cosubstrates and a multifunctional subunit." Faseb J 3(5): 1609-17. Koivu, J., R. Myllyla, et al. (1987). "A single polypeptide acts both as the beta subunit of prolyl 4-hydroxylase and as a protein disulfide-isomerase." J Biol Chem 262(14): 6447-9. Lamb, J. R., S. Tugendreich, et al. (1995). "Tetratrico peptide repeat interactions: to TPR or not to TPR?" Trends Biochem Sci 20(7): 257-9. Noiva, R. and W. J. Lennarz (1992). "Protein disulfide isomerase. A multifunctional protein resident in the lumen of the endoplasmic reticulum." J Biol Chem 267(6): 3553-6. Parkkonen, T., K. I. Kivirikko, et al. (1988). "Molecular cloning of a multifunctional chicken protein acting as the prolyl 4-hydroxylase beta-subunit, protein disulphide-isomerase and a cellular thyroid-hormone-binding protein. Comparison of cDNA-deduced amino acid sequences with those in other species." Biochem J 256(3): 1005-11. Pihlajaniemi, T., T. Helaakoski, et al. (1987). "Molecular cloning of the beta-subunit of human prolyl 4-hydroxylase. This subunit and protein disulphide isomerase are products of the same gene." Embo J 6(3): 643-9. Prockop, D. J. and K. I. Kivirikko (1995). "Collagens: molecular biology, diseases, and potentials for therapy." Annu Rev Biochem 64: 403-34. Roach, P. L., I. J. Clifton, et al. (1995). "Crystal structure of isopenicillin N synthase is the first from a new structural family of enzymes." Nature 375(6533): 700-4. |