 |
 |
 |
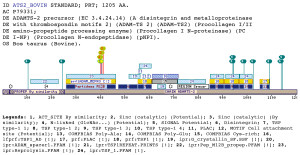
tsp1_adCell-cell and cell-extracellular matrix interactions are vital for the development and maintenance of an organism (Cal, Arguelles et al. 2001). In the same way, proteolysis of the extracellular matrix is very important for the tissue-remodeling processes occurring during both normal and pathological conditions. These events are mediated by a variety of cell surface adhesion proteins and proteases, with different structural and functional characteristics Among them, are the so called ADAM proteins that have raised considerable interest because of their potential ability to perform both functions, adhesion and proteolysis (Wolfsberg, Primakoff et al. 1995) (Blobel 1997). ADAMs are also associated with reproductive processes like spermatogenesis, sperm-egg binding and fusion (Wolfsberg and White 1996). Nevertheless, these are not the only functions of this kind of proteins, because they have been related to other processes such as myogenesis (Gilpin, Loechel et al. 1998) (5), osteoblast differentiation (6) (Inoue, Reid et al. 1998), and host defense (7) (Mueller, Rissoan et al. 1997). Furthermore, some ADAM family members, including TACE (tumor necrosis factor--converting enzyme), ADAM-12 (meltrin), and ADAM-23, have been suggested to play important roles in the development and progression of inflammatory and tumor processes (8-15) (Emi, Katagiri et al. 1993) (Kratzschmar, Lum et al. 1996).ADAMs are transmembrane proteins containing both a disintegrin and a metalloprotease domain (Wolfsberg, Straight et al. 1995). About two third of the proteins with an ADAM type metalloprotease domain also contain the zinc protease pattern which locates the active site of these proteases. As they contain an adhesion domain and a protease domain, ADAMs play an important role in adhesion and proteolityc processes. ADAMTS proteins (Tang and Hong 1999)constitute a related family of proteases. In these proteins, the metalloprotease and disintegrin domains are flanked by thrombospondin type I (TSP1) repeat. The proposed domain is always found in ADAM-TS family and usually lies between the TSP1 domain (PF00090) and right N-ter of the pfam :ADAM_spacer1 (PF05986). The TSP1 domain, also known as Thrombospondin type 1, is a repeat that was first described in 1986 by Lawler (Lawler and Hynes 1986). It was found in the thrombospondin protein where it is repeated 3 times. The number of proteins that posses this domain have grown substantially and several proteins involved in the complement pathway (properdin, C6, C7, C8A, C8B, C9) (Patthy 1988) as well as extracellular matrix protein like mindin, F-spondin (Feinstein, Borrell et al. 1999), SCO-spondin and even the circumsporozoite surface protein 2 and TRAP proteins of Plasmodium(Wengelnik, Spaccapelo et al. 1999) (Rogers, Rogers et al. 1992) contain one or more instance of this repeat. On the other hand, the ADAM_spacer1 represents the Spacer-1 region from the ADAM-TS family of metalloproteinases (Cal, Arguelles et al. 2001). The matched region was matched as a cysteine-rich region, but no more annotations have been made to this region. Examples


Taxonomic DistributionVertebrates: Homo sapiens, Pongo pygmaeus, Mus musculus, Rattus norvegicus, Gallus gallus. Insects: Drosophila melanogaster, Anopheles gambiae. Invertebrates: Caenorhabditis elegans, Caenorhabditis brigssae, Haemonchus contortus PSI-BLAST against ensembl (Apis mellifera)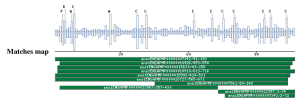
PSI-BLAST against ensembl (no taxonomic restriction)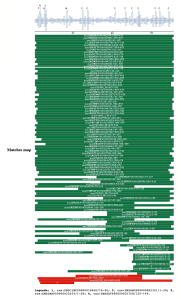
Comparison against UNIPROT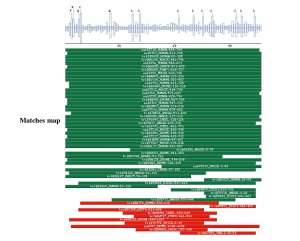
Retrived sequences in UNIPROT (txt format) Downloadable filesProfileMultiple Alignment PSI-BLAST against ensembl (Apis mellifera) PSI-BLAST against ensembl (no taxonomic restriction) REFERENCESBlobel, C. P. (1997). "Metalloprotease-disintegrins: links to cell adhesion and cleavage of TNF alpha and Notch." Cell 90(4): 589-92. Cal, S., J. M. Arguelles, et al. (2001). "Identification, characterization, and intracellular processing of ADAM-TS12, a novel human disintegrin with a complex structural organization involving multiple thrombospondin-1 repeats." J Biol Chem 276(21): 17932-40. Emi, M., T. Katagiri, et al. (1993). "A novel metalloprotease/disintegrin-like gene at 17q21.3 is somatically rearranged in two primary breast cancers." Nat Genet 5(2): 151-7. Feinstein, Y., V. Borrell, et al. (1999). "F-spondin and mindin: two structurally and functionally related genes expressed in the hippocampus that promote outgrowth of embryonic hippocampal neurons." Development 126(16): 3637-48. Gilpin, B. J., F. Loechel, et al. (1998). "A novel, secreted form of human ADAM 12 (meltrin alpha) provokes myogenesis in vivo." J Biol Chem 273(1): 157-66. Inoue, D., M. Reid, et al. (1998). "Cloning and initial characterization of mouse meltrin beta and analysis of the expression of four metalloprotease-disintegrins in bone cells." J Biol Chem 273(7): 4180-7. Kratzschmar, J., L. Lum, et al. (1996). "Metargidin, a membrane-anchored metalloprotease-disintegrin protein with an RGD integrin binding sequence." J Biol Chem 271(9): 4593-6. Lawler, J. and R. O. Hynes (1986). "The structure of human thrombospondin, an adhesive glycoprotein with multiple calcium-binding sites and homologies with several different proteins." J Cell Biol 103(5): 1635-48. Mueller, C. G., M. C. Rissoan, et al. (1997). "Polymerase chain reaction selects a novel disintegrin proteinase from CD40-activated germinal center dendritic cells." J Exp Med 186(5): 655-63. Patthy, L. (1988). "Detecting distant homologies of mosaic proteins. Analysis of the sequences of thrombomodulin, thrombospondin complement components C9, C8 alpha and C8 beta, vitronectin and plasma cell membrane glycoprotein PC-1." J Mol Biol 202(4): 689-96. Rogers, W. O., M. D. Rogers, et al. (1992). "Characterization of the gene encoding sporozoite surface protein 2, a protective Plasmodium yoelii sporozoite antigen." Mol Biochem Parasitol 53(1-2): 45-51. Tang, B. L. and W. Hong (1999). "ADAMTS: a novel family of proteases with an ADAM protease domain and thrombospondin 1 repeats." FEBS Lett 445(2-3): 223-5. Wengelnik, K., R. Spaccapelo, et al. (1999). "The A-domain and the thrombospondin-related motif of Plasmodium falciparum TRAP are implicated in the invasion process of mosquito salivary glands." Embo J 18(19): 5195-204. Wolfsberg, T. G., P. Primakoff, et al. (1995). "ADAM, a novel family of membrane proteins containing A Disintegrin And Metalloprotease domain: multipotential functions in cell-cell and cell-matrix interactions." J Cell Biol 131(2): 275-8. Wolfsberg, T. G., P. D. Straight, et al. (1995). "ADAM, a widely distributed and developmentally regulated gene family encoding membrane proteins with a disintegrin and metalloprotease domain." Dev Biol 169(1): 378-83. Wolfsberg, T. G. and J. M. White (1996). "ADAMs in fertilization and development." Dev Biol 180(2): 389-401. |