 |
 |
 |
A domain specific to the N-acetylgalactosaminyltransferases familyThe profile matches the N-acetylgalactosaminyltransferase family. The group of UDP-GalNAc:polypeptide N-acetylgalactosaminyltransferases (ppGaNTases) comprises a large family of metal-dependent enzymes that initiate mucin-type O-glycosylation. During catalysis, GalNAc is transferred from UDP-GalNAc to selected serine and threonine residues of proteins destined for the extracellular environment. The family of ppGaNTases is well- conserved during evolution (Hagen and Nehrke 1998).All known ppGaNTases contain such a DXH or DXD-like motif. Based on fold recognition, the DXD motif of the a1,3-fucosyltransferase is predicted to correspond to aspartate 100 in the active site of the bacteriophage T4 DNA b-glucosyltransferase (Wiggins and Munro 1998). Mutagenesis of aspartate residues in other DXD-containing enzymes (mannosyltransferase, glucosyltransferase, and chitin synthase 2) have shown that these residues are essential to catalytic activity (Busch, Hofmann et al. 1998) (Wiggins and Munro 1998) (Nagahashi, Sudoh et al. 1995). Threading analysis have suggested that the central 340-amino acid region of mammalian and Caenorhabditis elegans ppGaNTases share a common structural fold that consists of two domains, each of which contains a parallel b-sheet, flanked by a-helices. This fold most closely resembles the lac repressor protein, a member of the periplasmic binding protein family fold, which itself is structurally related to bacteriophage T4 DNA b-glucosyltransferase. Site directed mutagenesis have been used to mutate highly conserved and invariant aspartate, glutamate, and histidine residues and find that those residues required for enzymatic activity are predicted to line the active site cleft of a model based on the lac repressor crystal structure (Hagen, Hazes et al. 1999). No domains have been suggested for the region matched by the profile proposed by us, which lies between the Glycosyl transferase (IPR001173) domain and the Ricin-type beta-trefoil lectin domain. The Glycosyl transferase (IPR001173) is a diverse family, transferring sugar from UDP-glucose, UDP-N-acetyl- galactosamine, GDP-mannose or CDP-abequose, to a range of substrates including cellulose, dolichol phosphate and teichoic acids. The biosynthesis of disaccharides, oligosaccharides and polysaccharides involves the action of hundreds of different glycosyltransferases. These are enzymes that catalyse the transfer of sugar moieties from activated donor molecules to specific acceptor molecules, forming glycosidic bonds. A classification of glycosyltransferases using nucleotide diphospho-sugar, nucleotide monophospho-sugar and sugar phosphates and related proteins into distinct sequence based families has been described (Campbell, Davies et al. 1997) On the other hand the Ricin-type beta-trefoil lectin domain (PF00652) has been described as a member of the Beta-trefoil superfamily clan. Primary structure analysis has shown the presence of a similar domain in many carbohydrate-recognition proteins like plant and bacterial AB-toxins, glycosidases or proteases (Hirabayashi, Dutta et al. 1998), (Hazes and Read 1995) (Hazes 1996). This domain, known as the ricin B lectin domain, can be present in one or more copies and has been shown in some instance to bind simple sugars, such as galactose or lactose. Examples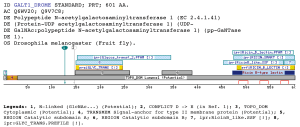
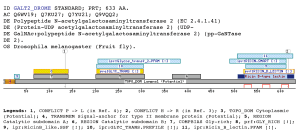
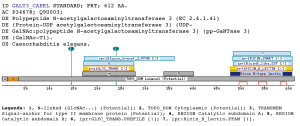
Taxonomic DistributionInsecta: Drosophila melanogaster. Vertebrata: Homo sapiens, Mus musculus, Bos taurus. Invertebrata: Caenorabditis elegans . PSI-BLAST against ensembl (Apis mellifera)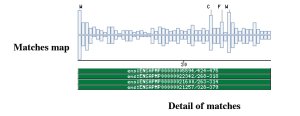
PSI-BLAST against ensembl (no taxonomic restriction)
Comparison against UNIPROT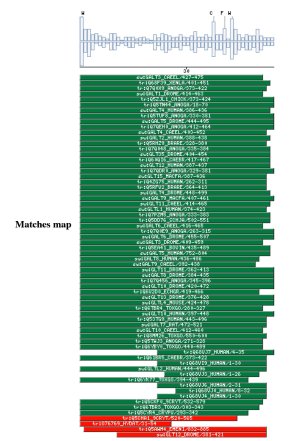
Retrived sequences in UNIPROT (txt format) Downloadable filesProfile Multiple Alignment PSI-BLAST against ensembl (Apis mellifera) PSI-BLAST against ensembl (no taxonomic restriction) REFERENCESBusch, C., F. Hofmann, et al. (1998). "A common motif of eukaryotic glycosyltransferases is essential for the enzyme activity of large clostridial cytotoxins." J Biol Chem 273(31): 19566-72. Campbell, J. A., G. J. Davies, et al. (1997). "A classification of nucleotide-diphospho-sugar glycosyltransferases based on amino acid sequence similarities." Biochem J 326 (Pt 3): 929-39. Hagen, F. K., B. Hazes, et al. (1999). "Structure-function analysis of the UDP-N-acetyl-D-galactosamine:polypeptide N-acetylgalactosaminyltransferase. Essential residues lie in a predicted active site cleft resembling a lactose repressor fold." J Biol Chem 274(10): 6797-803. Hagen, F. K. and K. Nehrke (1998). "cDNA cloning and expression of a family of UDP-N-acetyl-D-galactosamine:polypeptide N-acetylgalactosaminyltransferase sequence homologs from Caenorhabditis elegans." J Biol Chem 273(14): 8268-77. Hazes, B. (1996). "The (QxW)3 domain: a flexible lectin scaffold." Protein Sci 5(8): 1490-501. Hazes, B. and R. J. Read (1995). "A mosquitocidal toxin with a ricin-like cell-binding domain." Nat Struct Biol 2(5): 358-9. Hirabayashi, J., S. K. Dutta, et al. (1998). "Novel galactose-binding proteins in Annelida. Characterization of 29-kDa tandem repeat-type lectins from the earthworm Lumbricus terrestris." J Biol Chem 273(23): 14450-60. Nagahashi, S., M. Sudoh, et al. (1995). "Characterization of chitin synthase 2 of Saccharomyces cerevisiae. Implication of two highly conserved domains as possible catalytic sites." J Biol Chem 270(23): 13961-7. Wiggins, C. A. and S. Munro (1998). "Activity of the yeast MNN1 alpha-1,3-mannosyltransferase requires a motif conserved in many other families of glycosyltransferases." Proc Natl Acad Sci U S A 95(14): 7945-50. |