 |
 |
 |
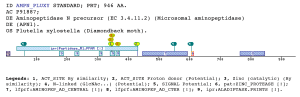
The M1 aminopeptidases case (AMINOPEP_AD_CENTRAL, AMINOPEP_AD_CTER)Metalloaminopeptidases remove the N-terminal amino acid from proteins or small oligopeptides. This modification process can occur either co-translationally or posttranslationally after the action of an endoproteinase.The metalloaminopeptidases utilize conserved amino acid residues to generate a scaffold capable of binding one or two metal ions (Lowther and Matthews 2002). The majority of zinc-dependent metallopeptidases (with the exception of the carboxypeptidases) share a common pattern of primary structure in the region of their sequence involved in the binding of zinc (Rawlings and Barrett 1995), and can be grouped together as a superfamily, known as the metzincins, on the basis of this sequence similarity. They can be classified into a number of distinct families (Rawlings, Tolle et al. 2004). The domains and profiles importance is that one of these families, the M1 family has been matched by two new profiles developed by us. The AMINOPEP_AD_CENTRAL is located toward the middle zone of the protein between the M1 peptidases family domain (IPR001930) and the AMINOPEP_AD_CTER domain, without overlapping with these two domains. In the other hand, the AMINOPEP_AD_CTER is located toward the protein C-terminus, which coincides with the Metallopeptidase domain terminus. Aminopeptidase N, is a membrane-bound metalloprotease, shown to be identical to CD13, a 150-kDa cell surface glycoprotein which serves as a receptor for serologically related coronaviruses of humans, pigs and cats. Furthermore, catalyzes the removal of single amino acids from the amino terminus of small peptides and probably plays a role in their final digestion (Wentworth and Holmes 2001) (Dan, Tani et al. 2003). CD13/aminopeptidase N is widely distributed in a variety of mammalian cells such as monocytes/macrophages, fibroblasts, neutrophils, endothelial cells, and epithelial cells, associated with the capability of migration of the cells (Kehlen, Lendeckel et al. 2003). Furthermore, is the major receptor for human coronavirus and gastroenteritis virus (Miguel, Pharr et al. 2002). Certain differences in glycosylation between coronavirus receptors from different species and within this domain are critical determinants in the species specificity of coronaviruses infection (Wentworth and Holmes 2001). Within the C-terminal aminopeptidase domain are present a glycosilation sequon that determines receptor activity for porcine and feline coronaviruses. Examples


Taxonomic DistributionVertebrates: Felis silvestris catus, Sus scrofa, Rattus norvegicus Archaea: Thermoplasma volcanium, Sulfolobus tokodaii, Sulfolobus solfataricus Fungi: Saccharomyces cerevisiae, Ashbya gossypii, Candida glabrata Bacteria: Lactococcus lactis, Streptomyces lividans, Staphylococcus epidermidis Insecta: Heliothis virescens, Manduca sexta, Lymantria dispar Invertebrates: Caenorhabditis briggsae Plants: Oryza sativa, Arabidopsis thaliana Protozoa: Plasmodium yoelii yoelii,Encephalitozoon cuniculi
REFERENCESDan, H., K. Tani, et al. (2003). "CD13/aminopeptidase N in collagen vascular diseases." Rheumatol Int 23(6): 271-6.Kehlen, A., U. Lendeckel, et al. (2003). "Biological significance of aminopeptidase N/CD13 in thyroid carcinomas." Cancer Res 63(23): 8500-6. Lowther, W. T. and B. W. Matthews (2002). "Metalloaminopeptidases: common functional themes in disparate structural surroundings." Chem Rev 102(12): 4581-608. Miguel, B., G. T. Pharr, et al. (2002). "The role of feline aminopeptidase N as a receptor for infectious bronchitis virus. Brief review." Arch Virol 147(11): 2047-56. Rawlings, N. D. and A. J. Barrett (1995). "Evolutionary families of metallopeptidases." Methods Enzymol 248: 183-228. Rawlings, N. D., D. P. Tolle, et al. (2004). "MEROPS: the peptidase database." Nucleic Acids Res 32 Database issue: D160-4. Wentworth, D. E. and K. V. Holmes (2001). "Addition of a single glycosylation site to hAPN blocks human coronavirus-229E receptor activity." Adv Exp Med Biol 494: 199-204. Wentworth, D. E. and K. V. Holmes (2001). "Molecular determinants of species specificity in the coronavirus receptor aminopeptidase N (CD13): influence of N-linked glycosylation." J Virol 75(20): 9741-52. |